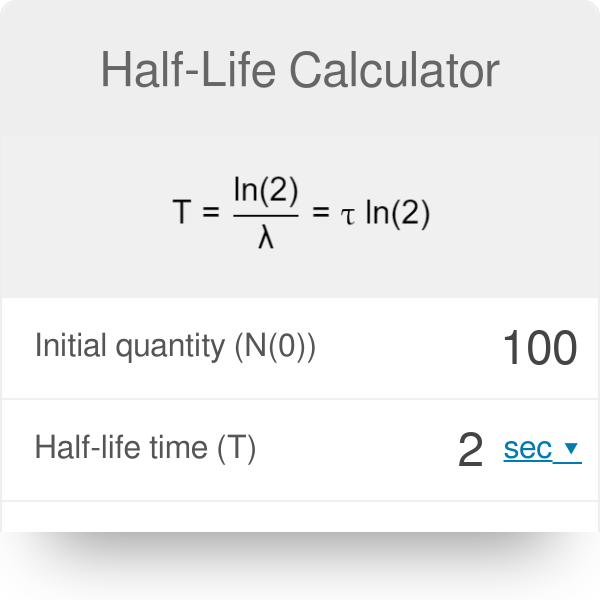
half life formula precalc
T 12 ln2λ. N t 15625 g A radioactive isotope will remain 15625 grams after 30 years if its half-life is 6 years and initial values are 500 grams.

How To Find Half Life Or Doubling Time Precalculus Study Com
The mathematical representation of Half life is given by Half life time Napierian logarithm of 2disintegration constant The equation is.
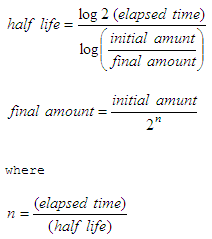
. I tried doing this. You can find the half-life of a radioactive element using the formula. A radioactive isotope of selenium 75 Se used in the creation of medical images of the pancreas has a half-life of 11977 days.
Half Life Precalc If Pharaoh Ramses II died in the year 1213 BC then what percent of the carbon-14 was left in the mummy of Ramses II in the year 2000. Write the equation of half-life and substitute the values. Quantity remains N t.
Given the half-life find the decay rate. How to solve for the half-life of any substance. The half-life eqt_ 12 eq of a substance with decay constant eqlambda eq can be calculated as eqt_ 12 dfrac ln 2 lambda eq seconds.
Fill in any three to calculate the fourth value. Initial quantity N 0. Disintegration constant of the system.
This equation is used in the calculator when solving. Most noteworthy this shows that the rate of popcorn eating was not at a steady pace and that the half-life of popcorn is of 15. The half-life of a substance is the amount of time it takes for half of the substance.
You can replace the N with the activity Becquerel or a dose rate of a substance as long as you use the same units for N t and N 0. Solve the equation for the remaining quantity N t. 17Calculus Precalculus - Half-Life.
This gives us the half-life formula t l n 2 k t-fracmathrmlnleft2rightk t k ln 2 How To. Round your answer to two decimal places. The general equation with half life N t N 0 05 t T In which N 0 is the number of atoms you start with and N t the number of atoms left after a certain time t for a nuclide with a half life of T.
If 250 milligrams are given to a patient how many milligrams A are left after 30 days. The time required for the original quantity to decay to half its amount is called the half-time. Where t12 is the half-life of the particle t is the elapsed time N0 is the quantity in the beginning and Nt is the quantity at time t.
After simplifying these values we will get. So when were dealing with half life specifically instead of exponential decay in general we can use this formula we got from substituting yC2. Half life h 5730 Time 2000 - 1213 t 787 And the equation I used was C12th I tried setting C 1213 since that was the year he died and it equals C when you set t 0 corresponding to the year.
Half-life is the time required for the amount of something to fall to half its initial value. T_frac 12 frac ln2 k where k is the decay constant. Example from class dealing with the 12 life example.

13 Exponentials 09 Askey Physics

Half Life Examples Using Exponent Laws To Solve Youtube
Exponential Growth And Decay College Algebra

Constructing Exponential Models Half Life Video Khan Academy
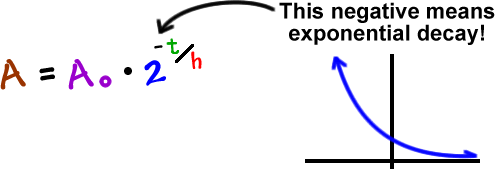
Exponentials Logarithms Cool Math Algebra Help Lessons Radioactive Decay And Decibel Levels
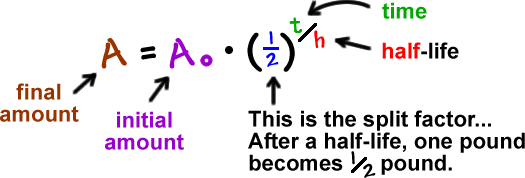
Exponentials Logarithms Cool Math Algebra Help Lessons Radioactive Decay And Decibel Levels

Precalculus Spring Half Life Problem Youtube

Doubling Time And Half Life Of Exponential Growth And Decay Math Insight
![]()
Formula Memorization Sheet Precalculus I Math 121 Study Notes Pre Calculus Docsity

Exponential Growth And Decay Word Problems Functions Algebra Precalculus Youtube

Doubling Time And Half Life Of Exponential Growth And Decay Math Insight

Precalculus Fall 2016 Acp Preview Problem 25 Half Life Equation Setup Problem Youtube
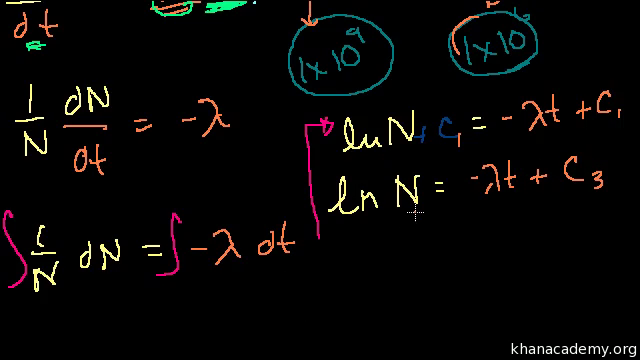
Exponential Decay Formula Proof Can Skip Involves Calculus Video Khan Academy

Solving Half Life Problems Youtube

Pre Calc 3 5 Half Life Youtube

Solving Half Life Problems How To Calculate Half Life 6 Otosection

Section 1 7 1 Doubling Time And Half Life Formulas Youtube